Compared to S-adenosylmethionine:
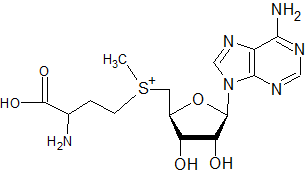
The following molecule would not be expected to act as a substrate in methylation (of DNA, rRNA, mRNA caps, tRNA, proteins, and phospholipids) or the transfer of propylamine, but not interfere with putrescine synthesis directly as sinefungin might:

This one could be able to catalyse the formation of spermidine and spermine, but lead to ethylation instead of methylation:

Whilst this molecule, if accepted by the respective enzymes as a substrate, could only be used for the transfer of propylamine but not for methylation/ethylation:

On the other hand, the following molecule would only act as a substrate for methylation and not for polyamine synthesis:

Whilst tubericidin is a SAM-analog that has been shown to affect L. donovani promastigotes but treatment has also led to tubericidin-resistant strains emerging:

In addition, attention should be paid to the stereoisomeric form of S-adenosylmethionine (AdoMet), the (Sc, Ss) isomer being the biologically active form, the (Sc, Rs) isomer being a potent inhibitor. (See review by Ronald Bentley).
Another idea that crossed my mind was whether methyl donor supplementation during pregnancy might render C57BL/6 or CBA mice more susceptible to leishmaniasis (similar to the results obtained for agouti mice regarding coat colour [Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003 Aug;23(15):5293-300.]).
No comments:
Post a Comment